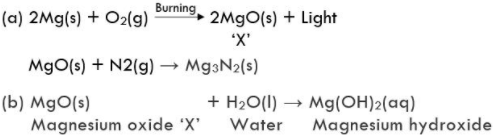NCERT Exemplar Solutions for Chapter 1 Chemical Reactions and Equations Class 10 Science
Short Answer Type Questions
1. Write the balanced chemical equation for the following equations for the following reaction and identify the type of reaction in each case.
(a) Nitrogen gas is treated with hydrogen gas in the presence of a catalyst at 773 K to form ammonia gas.
(b) Sodium hydroxide solution is treated with acetic acid to form sodium acetate and water.
(c) Ethanol is warmed with ethanoic acid to form ethyl acetate in presence of conc. H2SO4.
(d) Ethene is burnt in presence of oxygen to form carbon dioxide, water and releases heat and light.
Solution
![]()
It is a Combination reaction
(b) NaOH(aq) + CH3COOH(aq) → CH3COONa(aq) + H2O(l)
It is a Double displacement reaction/Neutralisation reaction. NaOH is a strong base and CH3COOH is a weak acid.
![]()
It is a Double displacement reaction/Esterification.
(d) C2H4(g) + 3O2(g) → 2CO2(g) + 2H2O(l) + heat + light
It is a Redox reaction/combustion reaction.
2. Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
(a) Thermite reaction, iron (III) oxide reacts with aluminium and gives molten iron and aluminium oxide. [CBSE 2012]
(b) Magnesium ribbon is burnt in an atmosphere of nitrogen gas to form solid magnesium nitride.
(c) Chlorine gas is passed in an aqueous potassium iodide solution to form potassium chloride solution and solid iodine.
(d) Ethanol is burnt in air to form carbon dioxide, water and releases heat.
Solution
(a) Fe2O3(s) + 2Al(s) → Al2O3(s)+ 2Fe(l) + heat
It is a displacement reaction because Al is displacing Fe from Fe2O3
(b) 3Mg(s)+ N2 → Mg3N2
It is a combination reaction as magnesium on burning reacts with N2 to form magnesium nitride.
(c) Cl2(g) + 2KI(aq) → 2KCl(aq) + I2(s)
It is a displacement reaction because chlorine is displacing iodine from potassium iodide to form potassium chloride and solid iodine.
(d) C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O + heat
It is oxidation reaction. It is also called combustion reaction.
3. Complete the missing components/variables given as x and y in the following reactions:
(a) Pb(NO3)2(aq) + 2Kl(aq) → PbI2(x) + 2KNO3(y)
(b) Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + x
(c) Zn(s) + H2SO4(aq) → ZnSO4(x) + H2O(y)
(d) CaCO3(s) ![]() CaO(s) + CO2(g)
CaO(s) + CO2(g)
Solution
(a) Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq)
(b) Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
(c) Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
![]()
Missing components/Variables
(i) x(s), y (aq)
(ii) x is 2Ag
(iii) x(aq), y(g)
(iv) x is heat
4. Which among the following changes are exothermic or endothermic in nature?
(a) Decomposition of ferrous sulphate.
(b) Dilution of sulphuric acid.
(c) Dissolution of sodium hydroxide in water.
(d) Dissolution of ammonium chloride in water.
Solution
(a) It is endothermic reaction.
(b) It is exothermic process.
(c) It is exothermic process.
(d) It is endothermic process.
5. Write the balanced chemical equations for the following reactions.
(a) Sodium carbonate on reaction with hydrochloric acid in equal molar concentrations gives sodium chloride and sodium hydrogen carbonate.
(b) Sodium hydrogencarbonate on reaction with hydrochloric acid gives sodium chloride, water and liberates carbon dioxide.
(c) Copper sulphate on treatment with potassium iodide precipitates cuprous iodide (Cu2I2), liberates iodine gas and also forms potassium sulphate.
Solution
(a) Na2CO3(s) + HCl(aq) → NaCl(aq) + NaHCO3(aq)
(b) NaHCO3(s) + HCl(aq) → NaCl(aq) + H2O(l) + CO2(g)
(c) 2CuSO4(aq) + 4Kl(aq) → 2K2SO4(aq) + Cu2I2(s) + I2(g)
6. Identify the reducing agent in the following reactions:
(a) 4NH3 + 5O2 → 4NO + 6H2O
(b) H2O + F2 → HF + HOF
(c) Fe2O3 + 3CO → 2Fe + 3CO2
(d) 2H2 + O2 → 2H2O
Solution
(a) NH3 is a reducing agent as it gives hydrogen to O2.
(b) F2 is a reducing agent.
(c) CO (Carbon monoxide) is a reducing agent.
(d) H2 is a reducing agent.
7. Identify the oxidising agent (oxidant) in the following reactions:
(a) Pb3O4 + 8HCl → 3PbCl2 + Cl2 + 4H2O
(b) 2Mg + O2 → 2MgO
(c) CuSO4 + Zn → Cu + ZnSO4
(d) V2O5 + 5Ca → 2V + 5CaO
(e) 3Fe + 4H2O → Fe3O4 + 4H2
(f) CuO + H2 → Cu + H2O
Solution
(a) Pb3O4 (Red lead). It is also called Sindur used by married ladies. It is an oxidising agent.
(b) O2 is oxidising agent.
(c) CuSO4 is oxidising agent.
(d) V2O5 is oxidising agent.
(e) H2O is oxidising agent.
(f) CuO is oxidising agent.
8. A solution of potassium chloride when mixed with silver nitrate solution, an insoluble white substance is formed. Write the chemical reaction involved and also mention the type of the chemical reaction.
Solution
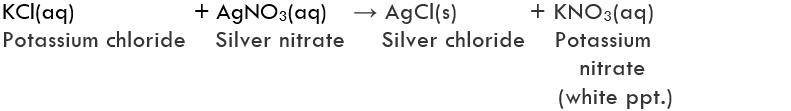
It is a double displacedment reaction. It is also a precipitation reaction as AgCl is a white precipitate.
9. Why do fireflies glow at night?
Solution
It is because protein present in fireflies undergoes oxidation in presence of air and an enzyme. This chemical reaction involves emission of visible light. Therefore, fireflies glow at night.
10. Grapes hanging on the plant do not ferment but after being plucked from the plant can be fermented. Under what conditions do these grapes ferment? Is it a chemical or a physical change?
Solution
After plucking grapes from plants, fermentation of sugar is carried out in the presence of yeast which changes sugar to ethanol and carbon dioxide. This process occurs in the absence of oxygen(anaerobic conditions).
Whereas grapes attach to plant involve in aerobic respiration and no fermentation can be possible under aerobic conditions.
Here fermentation is a chemical change as it results the formation of new substances; Alcohol and Carbon dioxide.
11. During the reaction of some metals with dilute hydrochloric acid, following observations were made.
(a) Silver metal does not show any change.
(b) The temperature of the reaction mixture rises when aluminium (Al) is added.
(c) The reaction of sodium metal is found to be highly explosive.
(d) Some bubbles of a gas are seen when lead (Pb) is reacted with the acid.
Explain these observations giving suitable reasons.
Solution
(a) It is because silver is less reactive than hydrogen. It cannot displace hydrogen from dilute acid.
(b) It is because the reaction is exothermic.
(c) Sodium is an alkali metal which is one of the most reactive metals and readily reacts with dilute HCl to form NaCl and hydrogen gas. The evolution of hydrogen gas cause explosion.
(d) Reaction of lead metal with dilute HCl forms lead (II) Chloride and releases hydrogen gas in the form of bubbles. Since reaction is quite slow due to less reactivity of lead, only bubbles of H2 are seen to evolve.
Pb + 2HCl(dil.) → PbCl2(s) + H2(g)
12. A substance ‘X’, which is an oxide of a group 2 element, is used intensively in the cement industry. This element is present in bones also. On treatment with water, it forms a solution which turns red litmus blue. Identify ‘X’ and also write the chemical reactions involved. [HOTS]
Solution
The substance X is calcium oxide (CaO) which is also known as quick lime. Reaction of quick lime with water forms calcium hydroxide. It is an alkaline solution and easily turns red litmus to blue.
Calcium oxide dissolves in water forming alkali which turns red litmus blue.

13. Write a balanced chemical equation for each of the following reactions and also classify them.
(a) Lead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic acid solution.
(b) A piece of sodium metal is added to absolute ethanol to form sodium ethoxide and hydrogen gas.
(c) Iron(III) oxide on heating with carbon monoxide reacts to form solid iron and liberates carbon dioxide gas.
(d) Hydrogen sulphide gas reacts with oxygen gas to form solid sulphuir and liquid water.
Solution
(a) Pb(CH3COO)2(aq) + 2HCl(dil) → PbCl2(s)↓ + 2CH3COOH (aq)
This is a double displacement and a precipitation reaction.
(b) 2C2H5OH(l) + 2Na(s) → 2C2H5O−Na+(aq) + H2(g)↑
This is a displacement reaction.
(c) Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)↑
This is an example of Redox reaction.
(d) 2H2S (g) + O2(g) → 2S(s) + 2H2O(l)
This is an example of Redox reaction.
14. Why do we store silver chloride in dark coloured bottles?
Solution
It is because silver chloride decomposes to silver and chlorine gas in presence of sunlight.
![]()
15. Balance the following chemical equations and identify the type of chemical reaction.
(a) Mg(s) + Cl2(g) → MgCl2(s)
Solution
(a) Mg(s) + Cl2(g) → MgCl2(s)
Combination reaction
(b) HgO(s) → 2Hg(l) + O2(g);
Decombination reaction
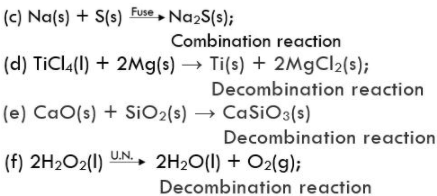
16. A magnesium ribbon is burnt in oxygen to give a white compound ‘X’ accompanied by emission of light. If the burning ribbon is now placed in an atmosphere of nitrogen, it continues to burn and forms a compound ‘Y’
(a) Write the chemical formulae of ‘X ’ and ‘Y’. [HOTS]
(b) Write a balanced chemical equation, when X is dissolved in water.
Solution
17. Zinc liberated hydrogen gas when reacted with dilute hydrochloric acid, whereas copper does not. Explain why
Solution
Zn(s) + 2HCl(dil.) → ZnCl2(aq) + H2(g)
Zinc is more reactive than H2, therefore, displace H2 from dil. HCl. Copper is less reactive than hydrogen, therefore, it does not liberate H2(g) from dilute acid.
18. A silver article generally turns black when kept in the open for a few days. The article when rubbed with toothpaste again starts shining.
(a) Why do silver articles turn black when kept in the open for a few days? Name the phenomenon involved.
(b) Name the black substance formed and give its chemical formula.
Solution
(a) Silver reacts with H2S gas present in atmosphere to form a black compound Ag2S (silver sulphide) on its surface. This phenomen on is called corrosion.
(b) Ag2S (silver sulphide) is a black coloured solid
Long Answer Type Questions
19. On heating blue coloured powder of copper (II) nitrate in boiling tube, copper oxide (black), oxygen gas and a brown gas ‘X’ is formed.
(a) Write a balanced chemical equation of the reaction.
(b) Identify the brown gas ‘X’ evolved.
(c) Identify the type of reaction.
(d) What could be the pH range of aqueous solution of the gas ‘X’?
Solution

(b) The brown gas ‘X ’ is nitrogen dioxide (NO2).
(c) The reaction is a thermal decomposition reaction.
(d) The gas ‘X ’ is acidic in nature because it is a non-metallic oxide. Its aqueous solution has a pH less than 7.
20. Give the characteristic tests for the following gases.
(a) CO2
(b) SO2
(c) O2
(d) H2
Solutions
(a) Pass the gas through limewater. Limewater turns milky which confirms the presence of CO2.
![]()
(b) The smell is the characteristic feature of SO2, which smells like a rotten egg.
(c) Bring a candle near oxygen gas. The intensity of candle flame is increased, it shows the presence of oxygen gas which is a supporter of combustion.
(d) Bring a burning matchstick near hydrogen gas. The gas will burn explosively with a pop sound. It confirms the presence of hydrogen.
21. What happens when a piece of
(a) zinc metal is added to copper sulphate solution?
(b) aluminium metal is added to dilute hydrochloric acid?
(c) silver metal is added to copper sulphate solution?
Also, write the balanced chemical equation if the reaction occurs.
Solution
(a) The solution will become colourless due to formation of zinc sulphate and reddish brown copper metal will get deposited
![]()
(b) Hydrogen gas and aluminium chloride solution will be formed.
2Al(s) + 6HCl(dil.) → 2AlCl3 + 3H2(g)
(c) No reaction will take place because silver is less reactive than copper, it cannot displace copper from copper sulphate.
22. What happens when zinc granules are treated with dilute solutions of H2SO4, HCl, HNO3, NaCl and NaOH? Also write the chemical equations if reaction occurs.
Solution
(1) When Zinc granules react with dil H2SO4 displacement reaction takes place leading to the formation of ZnSO4 liberating H2 gas
Zn(s)+ H2SO4(dil.) → ZnSO4(aq) + H2(g)
(2) When Zinc granules react with dil HCl displacement reaction takes place leading to the formation of ZnCl2 liberating H2 gas
Zn(s)+ 2HCl(dil.) → ZnCl2 (aq) + H2 (g)
(3) When Zinc granules react with dil HNO3 it leads to the formation of Zinc nitrate evolving H2O and nitrous oxide
3Zn(s)+ 8HNO3(dil.) → 3Zn(NO3)2(aq) + 2NO(g) + 4H2O(l)
(4) When Zinc granules react with NaCl there will not be any reaction. As sodium is more reactive than Zn and cannot replace by it.
(5) When Zinc granules react with NaOH solution
Zn(s)+ 2NaOH(aq) → Na2ZnO2(aq) + H2(g)
23. On adding a drop of barium chloride solution to an aqueous solution of sodium sulphite, white precipitate is obtained.
(a) Write a balanced chemical equation of the reaction involved.
(b) What other name can he given to this precipitation reaction?
(c) On adding dilute hydrochloric acid to the reaction mixture, white precipitate disappears. Why
Solution
![]()
(b) It is also called double displacement reaction.
(c) BaSO3(s) + 2HCl(dil.) → BaCl2(aq) + H2O(l) + SO2(g)
It is because barium sulphite reacts with HCl to form barium chloride which is soluble in water and liberates SO2(g).
24. You are provided with two containers made up of (A) copper and (B) aluminium. You are also provided with solutions of (a) dilute HCl (b) dilute HNO3 (c) ZnCl2 and (d) H2O. In which of the above containers these solutions can be kept?
Solution
A. When solutions are kept in copper container
(a) Dilute HCl: No reaction takes place as copper is less reactive than hydrogen. Therefore, dilute HCl can be kept in copper container.
(b) Dilute HNO3: Copper will react with dil. HNO3 as it is an oxidising agent and will form copper nitrate and nitrogen monoxide gas.
3Cu(s) + 8HNO3(dil.) → 3Cu(NO3)2(aq) + 2NO(g) + 4H2O(l)
Therefore, HNO3(dil.) cannot be kept in copper container.
(c) ZnCl2: No reaction takes place because copper is less reactive than Zn, therefore ZnCl2 can be kept in copper container.
(d) H2O : No reaction takes place because copper is less reactive than hydrogen. Therefore, water can be kept in copper container.
Thus, dil. HCl, ZnCl2 and H2O can be kept in copper container whereas dil. HNO3 cannot be kept in it.
B. When solutions are kept in aluminium container-
(a) Dilute HCl: Aluminium reacts with dil. HCl to form aluminium chloride and hydrogen gas is formed.
2Al(s) + 6HCl(dil.)→ 2AlCl3(aq.) + 3H2(g)
So, dil. HCl cannot be kept in aluminium container.
(b) Dilute HNO3: Aluminium gets oxidised by HNO3 to form a layer of aluminium oxide which does not react further therefore dil. HNO3 can be kept in aluminium container.
(c) ZnCl2: It reacts with Al to form aluminium chloride and zinc metal will get deposited.
3ZnCl2 + 2Al → 2AlCl3 + 3Zn
Therefore, Zinc chloride cannot be stored in aluminium container.
(d) H2O: It can be stored in aluminium container because aluminium does not react with cold as well as hot water.
Al reacts with steam to form aluminium oxide and hydrogen.
2Al (s) + 3H2O (g) → Al2O3(s) + 3H2(g)
Thus, dil. HNO3 and H2O can be kept in aluminium container whereas dil. HCl and ZnCl2 solution cannot be stored in it.